Food and Drug Administration (FDA) announced on December 19 that five medicines that are selling in the market have no medical registration when regular inspection was made.
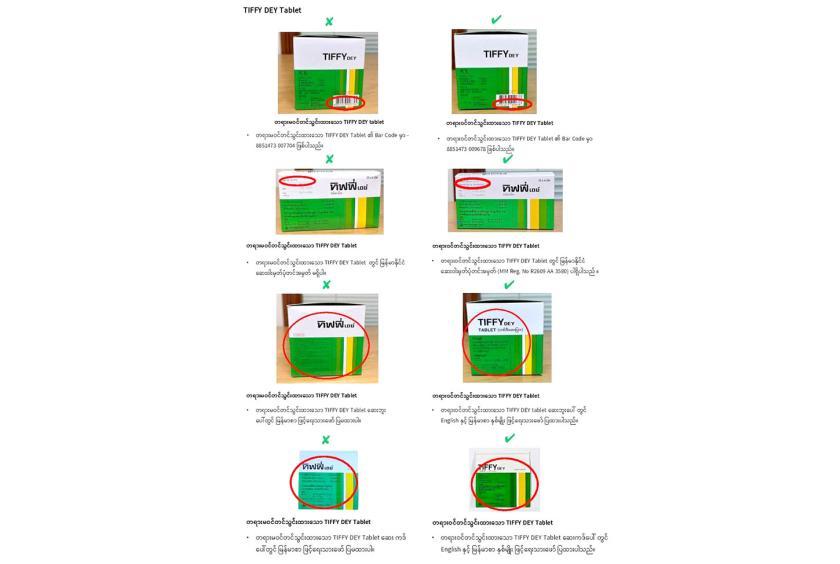
These unregistered medicines are Benda 500 Tablet, TIFFY DYE tablet, Sara Suspension (Strawberry), Sara for Children Suspension (Orange) and Avastin. In the domestic market, two types such as registered medicine and unregistered medicine are found. Therefore, comparison has been made for the public awareness, it said.
In the unregistered medicine, there are no Myanmar drug registration number, no description made in Myanmar language on the insert sheet and on the cover, and differences in Bar Code. FDA stated that unregistered medicines have no guarantee in quality, efficacy and safety and so public need to be cautious as they may be life threatening.
In October, FDA also announced 19 foodstuffs that need to be avoided which contain preservatives which aren’t allowed and use of unacceptable dye.